We employed new sulfur source, NaDDP to synthesis MoS2 microspheres through hydrothermal methods and investigated its photocatalytic activity of MoS2 for degradation of rhodamine B under various conditions.
Molybdenum disulfide (MoS2) is a layered semiconductor composed of three layers of covalently bonded S–Mo–S, and the three layers (S–Mo–S) can be arranged in the following way: hexagon (2H-MoS2), diamond (3R-MoS2) and triangle (1T-MoS2). However, the 2H-MoS2 is an indirect band gap semiconductor with a band gap in the range of 1.2–1.8 eV, which is an ideal visible light induced photocatalyst for high solar energy utilization efficiently.
The MoS2 microspheres were synthesized through hydrothermal methods by employing ammonium molybdate, sodium dialkyldithiophosphate and oxalic acid.
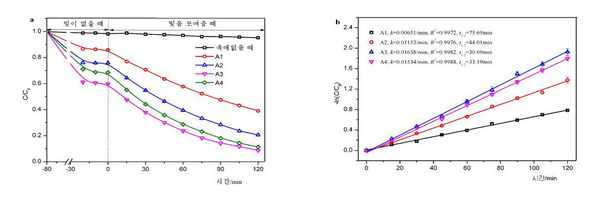
Figure 1 shows the photocatalytic activity of MoS2 microshperes under visible-light irradiation with the concentration of oxalic acid. During the 120 min reaction under visible-light irradiation, the dye degradation percentage varied according to the concentration of oxalic acid as follows: A3 (91.3%) > A4 (88.6%) > A2 (80.1%) > A1 (61.0%). The photocatalytic degradation of rhodamine B followed first-order kinetics. The rate constants for A1, A2, A3 and A4 were estimated to be 0.0065, 0.011, 0.016 and 0.015 min-1, respectively. These results showed that A3 exhibited the highest photocatalytic activity under visible-light irradiation.
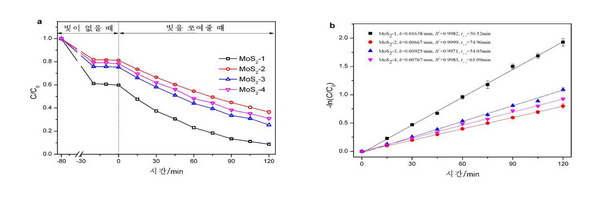
Fig. 2 shows the photocatalytic degradation of rhodamine B using MoS2 microshperes prepared at different sulfur sources: NaDDP(MoS2-1), thiourea(MoS2-2), thioaceteamide(MoS2-3) and L-cysteine(MoS2-4). 41.3% of rhodamine B was adsorbed onto MoS2-1, while 18.5, 27.3 and 21.2% of rhodamine B was adsorbed onto MoS2-2, MoS2-3 and MoS2-4, respectively. The degradation efficiency varied according to the sulfur sources as follows: MoS2-1 (91.3%) > MoS2-3 (81.3%) > MoS2-4 (77.1%) > MoS2-2 (70.0%). The rate constant (k) for MoS2-1, MoS2-2, MoS2-3 and MoS2-4 were estimated to be 0.01638, 0.00667, 0.00925 and 0.00767 min–1, respectively. These results showed that when NaDDP is used as a sulfur source, the best photocatalytic performance is achieved.
Our results of this study were published in Journal "Kinetics and Catalysts" under the title of "Photocatalytic Degradation of Rhodamine B on MoS2 Microspheres Prepared by Sodium Dialkyldithiophosphate-Assisted Hydrothermal Synthesis"(https://doi.org/10.1134/s00231-584-2303-0024).